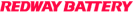Electrochemical Impedance Spectroscopy (EIS) is a powerful technique used in electrochemistry to study the response of an electrochemical system to sinusoidal perturbations in potential or current across a range of frequencies. Unlike Direct Current (DC) techniques, which focus on the system’s response over time, EIS allows researchers to investigate the system’s behavior across different frequencies, providing valuable insights into charge transfer, mass transport, and other electrochemical processes.
Question 1.
How is EIS related to Battery Screening and what role does it play in the process?
Electrochemical Impedance Spectroscopy (EIS) serves as a crucial tool in the realm of battery screening, offering insightful details on the electrochemical processes occurring within batteries. By employing advanced EIS battery cyclers, researchers and manufacturers can efficiently evaluate various performance parameters of batteries under different conditions. EIS plays a multifaceted role in the battery screening process, providing valuable information on the internal electrochemical behavior, impedance characteristics, and overall health of the batteries. Through sophisticated analysis techniques facilitated by EIS, such as impedance spectroscopy, researchers can gain a deep understanding of the battery’s performance, enabling them to make informed decisions about the suitability of batteries for specific applications. In essence, EIS contributes significantly to battery screening by offering a comprehensive assessment of electrochemical properties, aiding in the development of high-performing and reliable battery technologies.
Question 2.
What tools are available for drift correction in EIS measurements?
Drift correction in EIS measurements can be addressed using various tools that are available in EC-Lab. If you are encountering unusual or strange data at low frequencies while running EIS experiments on a corroding electrode or a charging/discharging battery, it is essential to ensure that any changes in your system do not interfere with your EIS measurements. To assist in managing such situations, EC-Lab provides a range of tools that can be utilized.
Question 3.
What are Total Harmonic Distortion (THD) and Non-Stationary Distortion (NSD) in EIS measurements?
In EIS measurements, Total Harmonic Distortion (THD) refers to the measure of non-linearity exhibited by a signal. It indicates the extent to which the signal deviates from being purely harmonic. On the other hand, Non-Stationary Distortion (NSD) in EIS measurements refers to the measure of non-stationarity in the signal, which signifies the degree to which the signal’s characteristics change over time rather than remaining constant. These parameters in EIS measurements play a crucial role in providing insights into the level of distortion and variability present in the signal being analyzed.
Question 4.
What is mass transport and what are the different mechanisms involved in it?
Mass transport is the process of matter displacement, which occurs through various mechanisms such as diffusion, convection, and migration. In diffusion, matter moves from areas of high concentration to areas of low concentration. Convection involves forced flow, where matter is transported through fluid movement. Migration refers to the movement of charged species under the influence of an electrical field. Two main mechanisms of mass transport are semi-infinite diffusion, occurring in electrolytes with a uniform concentration of electroactive species, and bounded diffusion (convection-diffusion), where mass transport involves a combination of convection and diffusion near the interface with a change in concentration of the electroactive species.
Question 5.
What is the role of Ohmic resistance in EIS and what components does it relate to?
Ohmic resistance plays a crucial role in Electrochemical Impedance Spectroscopy (EIS) as it represents the series resistance associated with various ohmic components within the system. These components include the electrolyte, electrical contacts, oxide layers, and any other elements that exhibit ohmic behavior. By measuring the ohmic resistance in EIS, researchers can assess the overall impedance of the system and gain insights into the individual contributions of these different components to the system’s overall resistance. Understanding the role of ohmic resistance and its relation to specific components allows for a more comprehensive analysis of the electrical behavior and characteristics of the system under investigation.
Question 6.
Why is linearity crucial for valid EIS measurements and interpretation?
Linearity is a critical aspect for conducting valid Electrochemical Impedance Spectroscopy (EIS) measurements and for accurately interpreting the data obtained from these measurements. In the context of EIS, linearity refers to the property of a system that follows the principle of superposition. This means that the system’s response to a sum of inputs is equal to the sum of its responses to each input individually. Maintaining linearity is essential for EIS measurements because it ensures that the behavior of the system under study can be accurately characterized and interpreted based on the relationships between the input and output signals.
In EIS, the ability to apply the principle of superposition relies on the system being linear. If the system is non-linear, it can lead to distorted or erroneous measurements, making it difficult to extract meaningful information about the underlying properties of the system being analyzed. Non-linear systems may exhibit complex interactions between different frequencies or amplitudes of the input signals, which can obscure the true impedance characteristics of the system.
By maintaining linearity in EIS measurements, researchers can confidently analyze impedance data and extract valuable insights about the electrical properties of the system under investigation. This ensures that the interpretations derived from EIS experiments are reliable and can be used to advance research in various fields, including electrochemistry, materials science, and engineering. Therefore, the presence of linearity in a system is crucial for obtaining accurate and interpretable results in Electrochemical Impedance Spectroscopy.
Question 7.
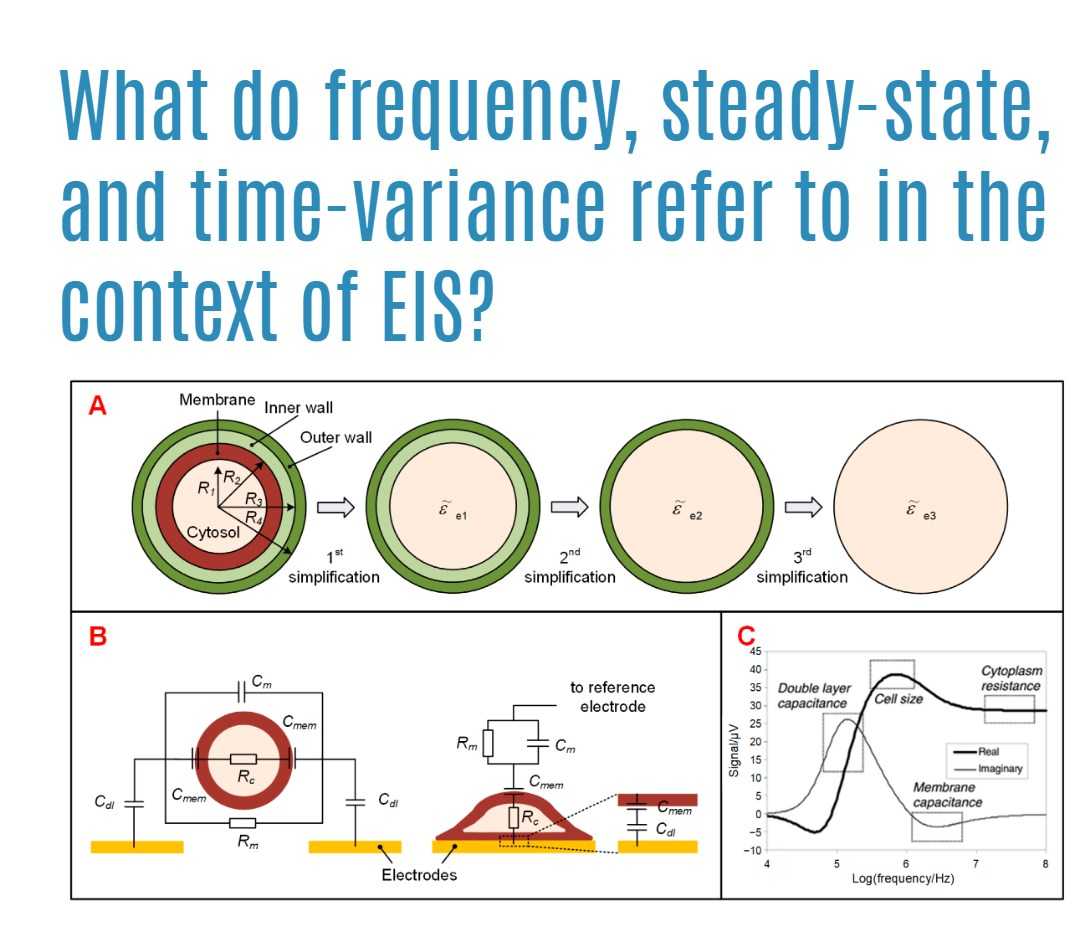
What do frequency, steady-state, and time-variance refer to in the context of EIS?
Frequency in the context of Electrochemical Impedance Spectroscopy (EIS) refers to the number of cycles of the electromagnetic wave per unit of time. It is a fundamental characteristic that plays a crucial role in understanding the response of systems under study. Steady-state, also known as a permanent state, is a condition reached after a sufficient amount of time has elapsed, where the system’s behavior becomes unchanging. Time-variance, on the other hand, indicates the property of a system where its parameters change with time. Understanding these terms is essential for accurate measurements and interpretation in EIS studies as they directly influence the behaviors observed in the system being analyzed.
Question 8.
What is the Faradaic impedance and how is it related to the electrochemical process?
Faradaic impedance is a measure that stems from the Faradaic current, which specifically pertains to the current associated with the electrochemical process taking place at the interface. In essence, Faradaic impedance is a representation of the resistance encountered by this specific current as it engages in the electrochemical reaction. It provides insight into the overall efficiency and effectiveness of the electrochemical process, as it reflects the obstacles that the current faces in its course. Therefore, the Faradaic impedance serves as a crucial metric for understanding and analyzing the electrochemical processes occurring at the interface.
Question 9.
What is an electrochemical interface and why is it important in EIS?
An electrochemical interface is established at the boundary between an electronic conductor, such as a metal or semi-conductor, and an ionic conductor, which could be in the form of a solid or a liquid electrolyte. This interface serves as the site where the crucial process of charge transfer takes place between the electronic and ionic conductors. In the context of Electrochemical Impedance Spectroscopy (EIS), understanding the electrochemical interface is fundamental because it plays a central role in determining the impedance behavior of the system under study. Monitoring how charge is transferred across this interface provides valuable insights into the electrical properties and kinetics of electrochemical processes occurring within the system. Therefore, the electrochemical interface is of great significance in EIS as it essentially governs the electrical behavior observed in these types of studies.
Question 10.
What is impedance and how is it defined in the context of EIS?
Impedance is a critical concept in the field of Electrochemical Impedance Spectroscopy (EIS). In EIS, impedance is defined as the frequency-dependent response of a system when it is subjected to an electrical disturbance. Specifically in the context of EIS, impedance can be understood as the transfer function of a system, with current as the input signal and potential as the output signal. This means that impedance quantifies how a system’s electrical properties respond to varying frequencies of an applied current signal, making it a valuable tool for analyzing and characterizing the electrical behavior of electrochemical systems.
Question 11.
What are the key electrical circuits commonly used in electrochemical impedance spectroscopy?
Key electrical circuits commonly used in electrochemical impedance spectroscopy include equivalent circuits that represent different electrochemical processes and interfaces. These circuits are essential for interpreting EIS measurements accurately.
– Butler-Volmer kinetics or Wagner-Traud circuit is used to model redox reactions without transport limitations, such as in Tafelian corrosion.
– The charge transfer and semi-infinite diffusion circuit are utilized for scenarios involving redox reactions or corrosion with mass-transport limitations, like electrode setups in quiescent electrolytes or aerobic corrosion conditions.
– The charge transfer and adsorption circuit, based on the Volmer-Heyrovský mechanism, are employed in applications like acidic corrosion, electrocatalysis, and electrolyzers.
– The charge transfer and convection-diffusion circuit are used in settings like redox reactions at Rotating Disk Electrodes or when redox reactions are controlled by the flow of electroactive species as seen in fuel cells and redox flow batteries.
– The charge transfer and restricted diffusion circuit model insertion reactions found in systems like lithium-ion batteries, permeation materials, and supercapacitors.
When studying complex systems like batteries, which involve multiple electrochemical interfaces, it is crucial to account for each interface by replicating the corresponding parts of the equivalent circuit related to Faradaic impedance. These circuits play a vital role in understanding the underlying electrochemical processes and behaviors in various devices and applications analyzed through electrochemical impedance spectroscopy.
Question 12.
How can Electrical Equivalent Circuit modeling help in interpreting EIS measurements?
Electrical Equivalent Circuit modeling is a valuable technique that can aid in interpreting Electrochemical Impedance Spectroscopy (EIS) measurements. By constructing an Electrical Equivalent Circuit (EEC) comprised of electrical and electrochemical elements like resistors, capacitors, inductors, and various diffusion types, researchers can create a model that simulates the behavior of the system being studied. This model is then compared to experimental impedance data using a fitting tool to determine parameter values that best align the simulated and experimental results. These parameters reflect the physical properties and processes occurring within the system.
In the context of EIS measurements, EEC modeling serves as a powerful tool for understanding the underlying mechanisms affecting the impedance response of the system. By accurately representing the system with an EEC, researchers can gain insights into the contribution of different components (such as resistive, capacitive, and inductive elements) to the overall impedance. This enables a more detailed analysis of the electrochemical processes, diffusion phenomena, and other factors influencing the measured impedance.
Moreover, specialized tools such as ZSim and ZFit, which are available in software interfaces like EC-Lab, offer functionalities for impedance simulation and fitting. These tools facilitate the process of comparing EEC models with experimental data, ensuring a close match that leads to meaningful parameter estimation. By utilizing these tools, researchers can extract valuable information regarding the system’s behavior, characterize its electrochemical properties, and ultimately improve their understanding of complex electrochemical systems.
Question 13.
How do I interpret my EIS measurements?
Interpreting electrochemical impedance spectroscopy (EIS) measurements involves understanding the complex electrochemical processes taking place at the interface of the electrode-electrolyte system and distinguishing these processes from electrical artifacts. EIS complements direct current (DC) techniques by providing a more comprehensive view of the system’s behavior over a wide frequency range, typically spanning from several megahertz to as low as 10 microhertz.
The interpretation of EIS data often involves constructing an Electrical Equivalent Circuit (EEC) that comprises both electrical components (such as resistors, capacitors, inductors, and constant-phase elements) and electrochemical components (including semi-infinite diffusion, restricted diffusion, and bounded diffusion). By fitting this circuit model to the experimental impedance data, one can extract valuable parameters that correspond to physical properties and processes within the system being studied.
It is essential to ensure that the impedance measurements are conducted under optimal conditions of linearity and stationarity to obtain accurate and meaningful results. The process of interpreting EIS measurements typically involves comparing the simulated impedance data generated from the EEC model with the experimental impedance data. By adjusting the parameters of the EEC model through a fitting tool to minimize the differences between the simulated and experimental data, researchers can gain insights into the underlying electrochemical and electrical phenomena occurring at the interface.
In summary, interpreting EIS measurements involves constructing an appropriate EEC model, fitting this model to the experimental data, and extracting meaningful information about the electrochemical processes and properties of the system under investigation.
Question 14.
What tools and methods are available to ensure accurate and reliable EIS measurements in systems that are not stationary or exhibit drift?
To ensure accurate and reliable Electrochemical Impedance Spectroscopy (EIS) measurements in systems that are not stationary or exhibit drift, several tools and methods are available. One key tool is the Non-Stationary Distortion (NSD) indicator, which helps check the stationarity of the system by analyzing the amplitudes of frequencies produced by the effect being assessed. Additionally, Total Harmonic Distortion (THD) is another important metric to consider, and more details on THD can be found in application notes and white papers.
When conducting EIS measurements at varying direct current (DC) voltages or currents, setting a waiting time to allow the input wave and system output to stabilize can be beneficial. It is also recommended to utilize the pw parameter to manage frequency transitions effectively.
For systems that are not stationary, especially when conducting low-frequency measurements, the drift correction tool can be utilized. This tool is explained in detail in specific application notes and helps account for drift in the system, leading to more accurate EIS measurements. However, it’s essential to note that enabling drift correction may extend the measurement time.
By incorporating these tools and methods, researchers can enhance the accuracy and reliability of EIS measurements, even in systems that are not stationary or exhibit drift. Furthermore, additional resources and information on Noise to Signal Ratio (NSR) can be beneficial in optimizing EIS measurements for various applications.
Question 15.
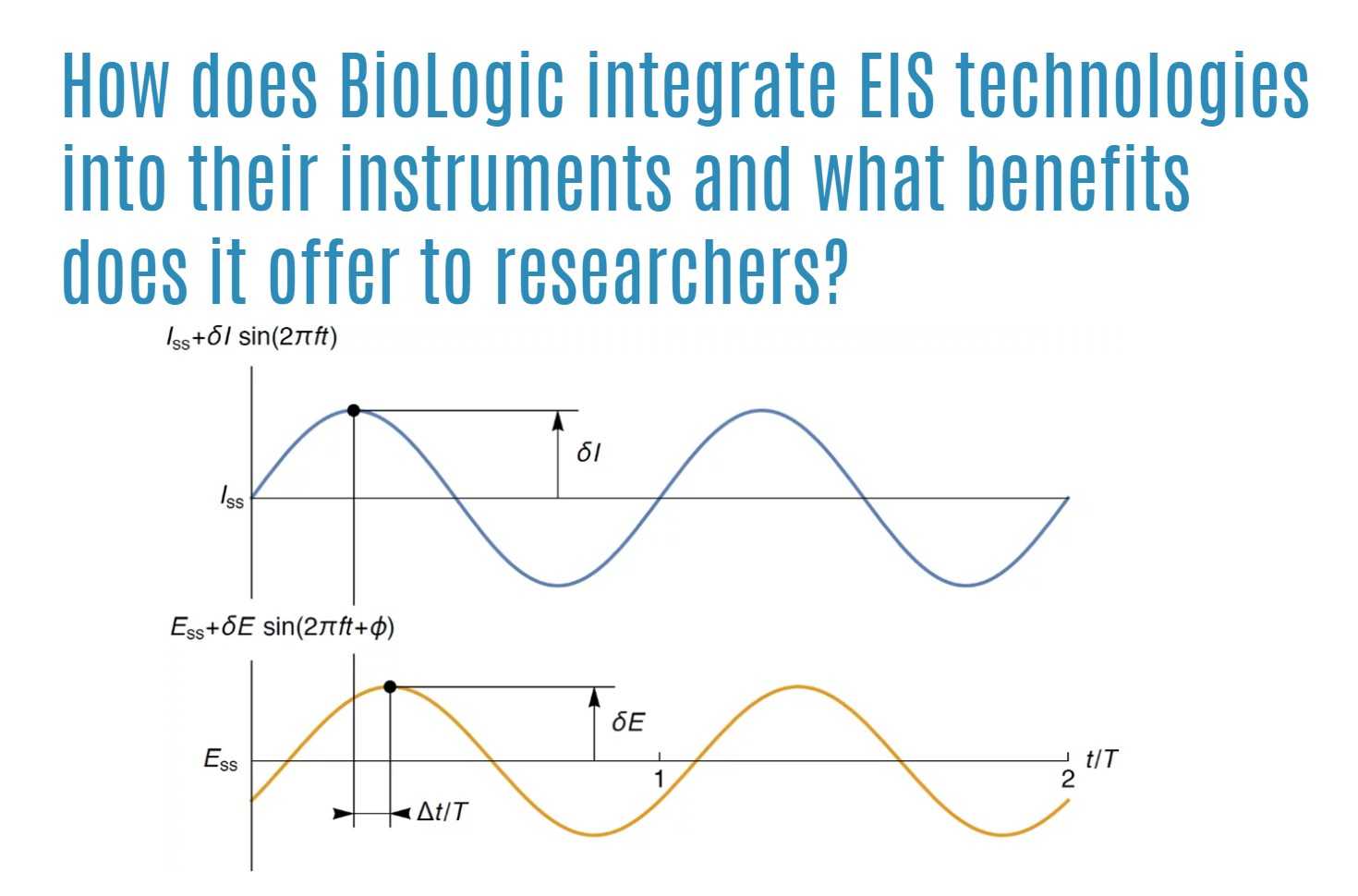
How does BioLogic integrate EIS technologies into their instruments and what benefits does it offer to researchers?
At BioLogic, a renowned authority in Electrochemical Impedance Spectroscopy (EIS) technologies, the goal is to democratize the accessibility of EIS for all researchers by embedding it into every instrument produced. By seamlessly integrating EIS functionality into their devices, BioLogic facilitates the smooth transition between AC and DC techniques. This integration allows researchers to utilize various control techniques, such as imposing AC sine waves on either a DC potential or a DC current value.
Moreover, BioLogic’s instruments offer additional techniques like SPEIS (Mott Schottky technique) and SGEIS, which enable applying sine waves on varied potentials or currents, respectively. The flexibility extends to sequenceable EIS techniques that can be implemented under different defined conditions throughout the frequency sweep. One of the key advantages provided by BioLogic’s instruments is the high level of accuracy maintained during EIS measurements. The error observed on the phase of the impedance module to magnitude is an important metric, with most potentiostats delivering precision within 1% and 1° accuracy below 500 kHz. Notably, the SP-300 potentiostat sets the bar even higher by offering unmatched performance with an accuracy of 0.3% and 0.3°. By incorporating EIS technology into their instruments and emphasizing accuracy and precision, BioLogic equips researchers with advanced tools to conduct thorough and intricate electrochemical impedance analyses with confidence and efficiency.
Question 16.
How is EIS used in research and industry applications?
Electrochemical impedance spectroscopy (EIS) is a powerful tool that can be leveraged to estimate the power delivery capability and state of health (SoH) of Li-ion batteries. Through EIS, rapid and accurate monitoring of the SoH of Li-ion batteries is facilitated, ultimately supporting the advancement of sustainable battery storage systems for electric vehicles (EVs) and grid-scale energy storage systems. Notably, EIS can be implemented without the need for disassembling the battery, enabling assessments to be conducted under real operating conditions. Currently, EIS is predominantly utilized for battery research and development initiatives, showcasing its potential to revolutionize the field. While the main focus of EIS remains on battery-related applications, its versatility extends to diverse sectors such as corrosion assessment, photovoltaics, fuel cell research, and even emerging applications in biology and medicine. By harnessing the capabilities of EIS across various industries and research domains, the potential for innovation and sustainable advancements is boundless.<
Related Posts
- Zapping the Voltage: A Simple Guide to Multimeter Testing for AAA Battery Voltage
- Will solid-state batteries replace lithium?
- Will Batteries Last Longer in the Freezer? Answers to Your Freezing Battery Myths!
- Will batteries last longer in the freezer?
- Why you shouldn’t charge your phone overnight?
- Why would someone put batteries in the freezer?