Welcome to the electrifying world of solid state batteries! These cutting-edge powerhouses are revolutionizing the way we think about energy storage. With their innovative design and advanced technology, solid state batteries are paving the way for a cleaner, more sustainable future. Let’s dive into the fascinating realm of solid state batteries and explore their types, benefits, applications, challenges, and what lies ahead in this dynamic field.
Types of Solid State Batteries
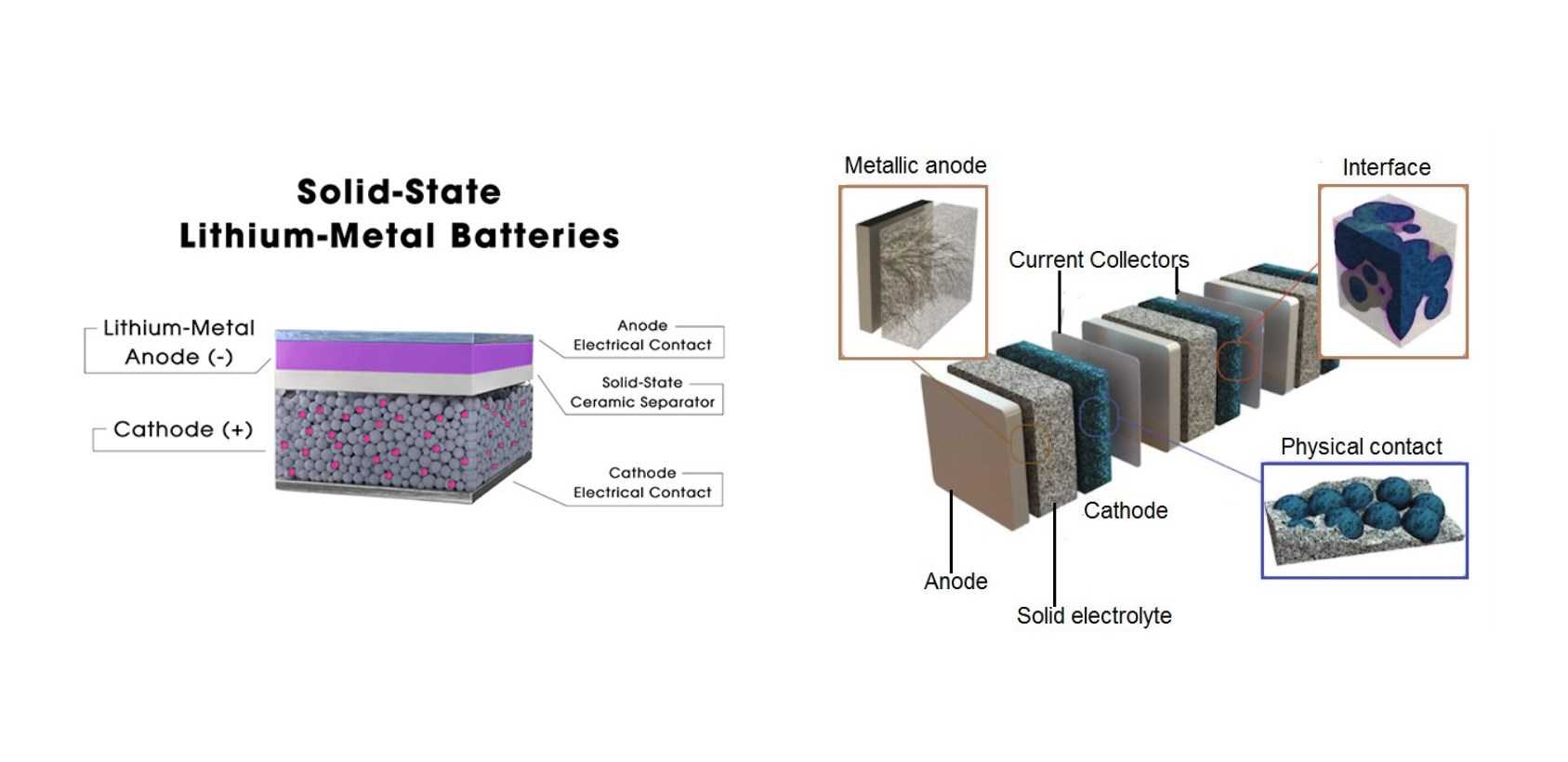
Solid state batteries come in various types, each with its unique characteristics and advantages. One type is the sulfide-based solid state battery, known for its high ionic conductivity. Another type is the oxide-based solid state battery, which offers stability and compatibility with different electrode materials.
Additionally, polymer-based solid state batteries are flexible and lightweight, making them suitable for wearable devices or applications where space is limited. Lithium metal solid state batteries are also gaining attention for their potential to increase energy density compared to traditional lithium-ion batteries.
Furthermore, ceramic-based solid state batteries are known for their safety features and resistance to dendrite formation. Each type of solid state battery has its own set of benefits and challenges that researchers continue to explore in the quest for more efficient and sustainable energy storage solutions.
Pros and Cons of Solid State Batteries
Solid state batteries offer several advantages over traditional lithium-ion batteries. One of the main pros is their higher energy density, which allows for longer-lasting battery life in electronic devices. Additionally, solid state batteries are safer to use since they don’t contain flammable liquid electrolytes like conventional batteries do.
Another benefit of solid state batteries is their faster charging capabilities and improved performance at extreme temperatures. This makes them ideal for use in electric vehicles and other high-demand applications where reliability is crucial. Furthermore, solid state batteries have a longer lifespan compared to traditional lithium-ion batteries, reducing the need for frequent replacements.
On the flip side, one of the cons of solid state batteries is their higher cost of production due to the use of more expensive materials and manufacturing processes. Additionally, current technology limitations make it challenging to scale up production to meet growing demand quickly.
Despite these drawbacks, ongoing research and development efforts aim to address these issues and further improve solid state battery technology in the future.
Applications of Solid State Batteries
Solid state batteries are revolutionizing various industries with their advanced technology and unique properties. One of the key applications of solid state batteries is in electric vehicles, where they offer higher energy density and improved safety compared to traditional lithium-ion batteries. This enables EVs to have longer driving ranges and faster charging times, making them more practical for everyday use.
In addition to transportation, solid state batteries are also finding applications in consumer electronics such as smartphones and laptops. Their compact size, lightweight design, and enhanced performance make them ideal for powering these devices efficiently. Furthermore, solid state batteries are being used in renewable energy storage systems to store excess energy generated from solar panels or wind turbines.
The aerospace industry is another sector benefiting from the use of solid state batteries due to their high energy density and reliability. Satellites, drones, and other space vehicles rely on these advanced power sources for long-duration missions without the need for frequent recharging. The versatile applications of solid state batteries continue to expand across various sectors, paving the way for a more sustainable future powered by innovative battery technology.
Challenges and Innovations in Solid State Battery Technology
Solid state batteries have shown great promise in revolutionizing the energy storage industry, but they are not without their challenges. One of the main obstacles faced by researchers and manufacturers is the high cost of production associated with solid state battery technology. Innovations in materials and manufacturing processes are needed to bring down costs and make these batteries more accessible to consumers.
Another challenge is related to the performance of solid state batteries at low temperatures. Unlike traditional lithium-ion batteries that can struggle in extreme cold conditions, solid state batteries need to maintain efficiency across a wider temperature range for widespread adoption. Researchers are exploring solutions such as new electrolyte formulations and improved electrode designs to address this issue.
Furthermore, ensuring the long-term stability and safety of solid state batteries remains a key focus area for innovation. The development of reliable solid electrolytes that can prevent dendrite formation and thermal runaway is crucial for enhancing the overall performance and safety of these advanced energy storage devices.
Comparison with Traditional Lithium-Ion Batteries
Solid state batteries have been gaining attention for their potential to outperform traditional lithium-ion batteries in various aspects. One key difference lies in the electrolyte material used: while lithium-ion batteries typically use liquid electrolytes, solid state batteries utilize solid electrolytes. This feature enhances safety by reducing the risk of leakage or combustion.
Moreover, solid state batteries offer higher energy density compared to conventional lithium-ion counterparts. This means that they can store more energy in a smaller and lighter package, making them ideal for applications where space and weight are critical factors.
In terms of lifespan, solid state batteries tend to have longer cycle lives than traditional lithium-ion batteries. This extended durability could translate into cost savings over time as replacements would be less frequent.
Despite these advantages, there are still challenges such as manufacturing costs and scalability that need to be addressed before solid state batteries can become widely adopted in commercial products. However, ongoing research and advancements suggest a promising future for this innovative battery technology.
Future Outlook for Solid State Batteries
The future outlook for solid state batteries is brimming with excitement and potential. As technology continues to advance, these innovative power sources are expected to revolutionize the way we use energy in various industries.
Researchers are tirelessly working on enhancing the performance and scalability of solid state batteries, aiming to make them more efficient and cost-effective. With ongoing developments in materials science and manufacturing processes, we can anticipate even smaller, lighter, and more powerful solid state battery solutions hitting the market in the near future.
Moreover, as concerns about environmental sustainability grow, solid state batteries offer a greener alternative to traditional lithium-ion batteries. Their non-flammable nature and longer lifespan make them an attractive option for electric vehicles, electronics, and renewable energy storage systems.
The trajectory of solid state battery technology points towards a brighter future where clean energy storage solutions play a pivotal role in shaping a sustainable world.
Conclusion
Solid state batteries represent the future of energy storage technology, offering significant advantages over traditional lithium-ion batteries. With their higher energy density, improved safety features, and potential for rapid charging capabilities, solid state batteries are set to revolutionize various industries from consumer electronics to electric vehicles.
As research and development in this field continue to advance, we can expect to see even more innovations that address current challenges such as manufacturing costs and performance optimization. The ongoing efforts towards commercializing solid state batteries will pave the way for a cleaner and more sustainable future.
Solid state batteries hold immense promise for transforming the way we power our devices and vehicles. As these cutting-edge battery technologies become more accessible and widespread, they have the potential to reshape the landscape of renewable energy storage and drive us towards a greener tomorrow.